Abstract
Introduction
Hepatic VOD/SOS is a progressive, potentially life-threatening complication early post-HSCT, or of nontransplant chemotherapy. VOD/SOS diagnosis has been based on Baltimore (≤21 days post-HSCT and bilirubin ≥2 mg/dL plus ≥2 of: hepatomegaly, ascites, weight gain ≥5%) or modified Seattle (≤20 days post-HSCT and ≥2 of: bilirubin >2 mg/dL, hepatomegaly or right upper quadrant pain, weight gain [>5% in defibrotide studies]) criteria. Recent European Society of Blood and Marrow Transplantation (EBMT) VOD/SOS guidelines require elevated bilirubin only for adults diagnosed ≤21 days post-HSCT (the literature suggests bilirubin <2 mg/dL before Day +21 is uncommon) but not for adults with late-onset (diagnosis >21 days post-HSCT) or pediatric patients (~30% of pediatric patients present with anicteric VOD/SOS [ie, bilirubin <2 mg/dL]). EBMT notes that hyperbilirubinemia may be a late finding in the progression of VOD/SOS. Defibrotide is approved to treat hepatic VOD/SOS with renal and/or pulmonary dysfunction post-HSCT in the United States and Canada, and to treat severe hepatic VOD/SOS post-HSCT in patients aged >1 month in the European Union. This post hoc analysis examines incidence of VOD/SOS without elevated bilirubin, and survival in defibrotide-treated, post-HSCT patients in the T-IND program (2007-2016).
Methods
Prior to US approval, defibrotide was available through the T-IND expanded-access program. The original protocol required VOD/SOS post-HSCT diagnosed per Baltimore criteria (which require hyperbilirubinemia) or biopsy, and multi-organ dysfunction (MOD). The protocol was amended to include patients without MOD (2009) and with VOD/SOS per modified Seattle criteria (which do not require hyperbilirubinemia; 2012). Patients received defibrotide 25 mg/kg/day (6.25 mg/kg q6h) recommended for ≥21 days.
Results
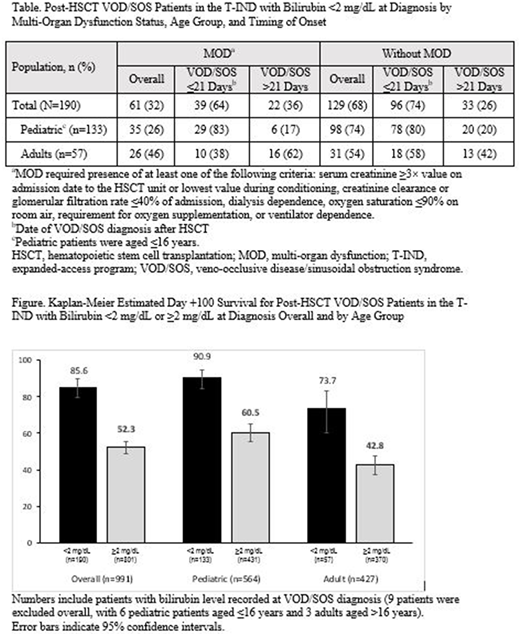
Of 991 patients in the T-IND with VOD/SOS post-HSCT and recorded bilirubin level at diagnosis, 190 (19%) had bilirubin <2 mg/dL (breakdown by subgroups in the Table), and 801 (81%) had bilirubin ≥2 mg/dL. Of those with bilirubin <2 mg/dL, 133 were pediatric patients aged ≤16 years (24% of all post-HSCT pediatric patients with recorded bilirubin [n=564]), and 57 were adult patients aged >16 years (13% of all post-HSCT adult patients with recorded bilirubin [n=427]). Diagnosis by Day +21 post HSCT (ie, not late onset) was recorded for 135/190 (71%) patients with bilirubin <2 mg/dL (107/133 [80%] pediatric patients; 28/57 [49%] adults).
In the overall post-HSCT group treated with defibrotide in the T-IND (n=1000; with and without elevated bilirubin at diagnosis, including 9 patients without bilirubin measurement at diagnosis), Kaplan-Meier estimated Day +100 survival was 58.9% (95% confidence interval [CI], 55.7%-61.9%). Kaplan-Meier estimated Day +100 survival was 85.6% (95% CI, 79.7%-89.9%) for the 190 patients with bilirubin <2 mg/dL at diagnosis and 52.3% (95% CI, 48.7%-55.7%) for the 801 patients with bilirubin ≥2 mg/dL (survival by age subgroups in the Figure). In the overall population of patients with bilirubin <2 mg/dL, 61.1% and 18.4% of patients had ≥1 treatment emergent adverse event (TEAE) and ≥1 treatment related adverse event (TRAE), respectively, and 21.1% had ≥1 hemorrhage event; for patients with bilirubin ≥2 mg/dL: 73.8% had ≥1 TEAE, 21.7% had ≥1 TRAEs, and 31.1% had ≥1 hemorrhage event.
Conclusions:
In the T-IND, 19% of post-HSCT patients with VOD/SOS had bilirubin <2 mg/dL at diagnosis, including 24% of children. Accordingly, 190 patients would not have been diagnosed if hyperbilirubinemia was a required criterion. Moreover, enrollment prior to 2012 required hyperbilirubinemia (or biopsy), so this percentage may understate the incidence of anicteric VOD/SOS. Of patients with bilirubin <2 mg/dL, 80% of pediatric patients and 49% of adults were diagnosed with VOD/SOS by Day +21 post-HSCT, suggesting that anicteric VOD/SOS may develop in this timeframe not only in pediatric patients but also in a sizeable number of adult patients. Defibrotide showed higher survival in patients with bilirubin <2 mg/dL compared to those with levels ≥2 mg/dL. These results compare favorably with the overall study findings, suggesting that treatment before the onset of hyperbilirubinemia may lead to better outcomes. The safety profile of the T-IND was similar to that of previous studies of defibrotide for the treatment of VOD/SOS.
Support: Jazz Pharmaceuticals.
Corbacioglu:Gentium: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria. Kernan:National Cancer Institute: Research Funding. Pagliuca:Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gentium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ryan:Jazz Pharmaceuticals: Employment, Other: Stock and stock options. Tappe:Jazz Pharmaceuticals: Employment, Other: Stock and stock options. Richardson:Karyopharm: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal